Abstract
Background: Itacitinib is a potent, selective oral Janus kinase (JAK) 1 inhibitor with broad anti-inflammatory activity. GRAVITAS-309 was designed as a 2-part, multicenter, randomized trial to assess the efficacy and safety of itacitinib in combination with corticosteroids (CS) as first-line treatment for moderate or severe chronic graft-versus-host disease (GVHD). We describe preliminary results from the open-label, dose-finding portion of the study.
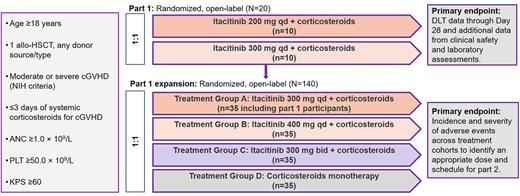
Methods: The study initially investigated itacitinib at doses of 200 mg once daily (qd; n=11) and 300 mg qd (n=10) in combination with CS. Both doses were well tolerated. The trial was then expanded to test itacitinib 400 mg qd and 300 mg twice daily (bid); the initial 300-mg qd cohort was expanded, and a CS monotherapy cohort was added (Figure 1). The expected CS starting dose was 0.5-1 mg/kg per day prednisone (or methylprednisolone equivalent) with a dose taper initiated on Day 14 or earlier during the second week of treatment and following published recommendations (Flowers and Martin. Blood. 2015;125[4]:606-15). Itacitinib dosage was initially reduced by 100 mg when given concomitantly with a strong CYP3A4 inhibitor (sCYP3A4i; eg, azole antifungals). A total of 140 patients (pts) (35/treatment group) with moderate or severe chronic GVHD were to be randomized 1:1 using chronic GVHD risk status (moderate vs severe) as a stratification factor. The primary objective was to identify the appropriate dose and schedule of itacitinib for further development. The data cutoff was January 31, 2022.
Results: 103 pts were enrolled and received at least one dose of itacitinib. The cohorts were well balanced for pt and disease characteristics. Median age was 57 years (range, 23-76) for the itacitinib cohorts and 59 years (range, 28-75) for the CS cohort. Approximately two-thirds of pts had moderate chronic GVHD. At the time of data cut, 39 (38%) pts in the itacitinib cohorts and 10 (28%) pts in the CS cohort continued study treatment. Median duration of study treatment was 27-28 weeks for the itacitinib cohorts (including 13-16 weeks of concomitant CS) and 17 weeks for the CS cohort. The itacitinib 300-mg bid dose cohort was discontinued early due to malignancy relapse occurring in 4/29 (14%) pts and more pts requiring dose reductions compared with the 400-mg qd and 300-mg qd cohorts (28% vs 17% and 8%, respectively), mostly due to cytopenias. Incidence of grade ≥3 treatment-emergent adverse events (AEs) was higher in the itacitinib cohorts than with CS (65% vs 31%) and were reported most commonly in the system organ classes of infections (44% vs 6%) and blood and lymphatic system disorders (23%-34% vs 6%). Cytomegalovirus (CMV) infections occurred in 10%-15% of pts in itacitinib cohorts (including CMV pneumonia [n=3], esophagitis [n=1], enteritis [n=1]) vs 3% with CS. The all-cause mortality rate was 14%-18% in itacitinib cohorts vs 11% with CS, with infections the leading cause.
The overall response rate (ORR) at 6 months was 46% (complete response [CR], 14%) with 300-mg qd and 53% (CR, 32%) with 400-mg qd, vs 36% (CR, 18%) with CS.
A preliminary exposure-response analysis showed a bell-shaped curve with maximum efficacy decreasing at higher exposures, consistent with increasing incidence of AEs. 60%-70% of patients received coadministration with a sCYP3A4i at any time point during study treatment, which increased itacitinib exposure across all doses, requiring dose reduction by 200 mg qd.
Conclusion: Preliminary results for first-line treatment of moderate or severe chronic GVHD showed an increase in ORR and CR rate with itacitinib + CS vs CS alone in both the 300-mg qd and 400-mg qd cohorts, at the expense of increased rates of cytopenias, infections, and mortality. This benefit:risk ratio does not support moving the combination of itacitinib + CS to a later phase of development in first-line therapy of chronic GVHD.
Disclosures
Im:Incyte: Research Funding; Abbvie: Consultancy; CTI Biopharma: Consultancy. Wolff:Incyte Corporation: Honoraria; Sanofi: Honoraria; Novartis: Honoraria, Research Funding; Behring: Honoraria. Cutler:Incyte Corporation: Consultancy, Honoraria; Equilium: Consultancy, Honoraria; Omeros: Consultancy, Honoraria; Mallinckrodt: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Jazz Educational: Consultancy, Honoraria; CTI Biopharma: Consultancy, Honoraria; CSL Behring: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Cimeio: Membership on an entity's Board of Directors or advisory committees; Oxford Immune Algorithmic: Membership on an entity's Board of Directors or advisory committees. Zeiser:Mallinckrodt: Honoraria, Speakers Bureau; Novartis Pharmaceuticals: Honoraria, Speakers Bureau; Incyte Corporation: Honoraria, Speakers Bureau. Shah:Novartis: Consultancy; Lilly Oncology: Consultancy, Honoraria; TG therapeutics: Consultancy; Kite Pharma: Consultancy; Miltenyi Biotec: Consultancy, Research Funding; Incyte Corporation: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy; Epizyme: Consultancy. Tan:Incyte Corporation: Consultancy. Ali:Abbvie: Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Law:Kite Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Atara: Research Funding; Sierra: Research Funding; Incyte Corporation: Research Funding; Jazz Educational: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Wagner:Novartis: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Other: Travel Grant; Amgen: Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees; Medac: Other: Travel Grant; Kite Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Ivanova:Incyte Corporation: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Hou:Incyte Corporation: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Bleam:Incyte Corporation: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Morariu-Zamfir:Incyte Corporation: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Pavletic:Center for Cancer Research at the National Cancer Institute: Other: Clinical Research Development Agreements with Celgene, Actelion, Eli Lilly, Pharmacyclics and Kadmon Corporation, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal